How to Ensure Effective Medical Device Testing and Compliance in 2023
In the rapidly evolving landscape of healthcare technology, Medical Device Testing has become a critical component in ensuring the safety and efficacy of new products. As we step into 2023, the urgency for rigorous testing protocols is greater than ever, necessitated by the increasing complexity of medical devices and the strict regulatory environments in which they operate. The introduction of innovative technologies, such as artificial intelligence and connected devices, has not only transformed patient care but has also presented unique challenges to manufacturers striving for compliance and safety.

Effective Medical Device Testing is essential not only for regulatory approval but also for building trust with healthcare professionals and patients. Companies must adopt robust testing methodologies that encompass a wide range of considerations, from biocompatibility and usability to performance under varied clinical scenarios. Furthermore, staying updated with the latest regulations and standards is imperative, as they continually evolve to meet the demands of an advancing industry. This introduction lays the groundwork for exploring strategies that can enhance testing protocols, promote compliance, and ultimately ensure that medical devices deliver safe and effective solutions for patients in need.
Overview of Medical Device Testing and Compliance Standards in 2023
In 2023, the landscape of medical device testing and compliance is heavily influenced by newly revised regulatory guidelines. For instance, the release of the registration review guidelines for intense pulsed light (IPL) therapy devices reflects the ongoing efforts to ensure safety and efficacy of such products. These guidelines categorize IPL systems, both regular and portable handheld hair removal devices, under specific classifications, allowing users to operate these devices independently according to prescribed instructions. This regulatory clarity is pivotal in streamlining the approval process and enhancing consumer confidence.
Moreover, the announcement of guidelines for other medical devices, such as drug-coated balloons and non-needle injectors, signifies a comprehensive approach to compliance standards across various categories. These documents serve as essential resources for manufacturers and stakeholders in the medical field, offering detailed pathways for product registration within classifications like neurological devices and ocular instruments. Each updated guideline not only adheres to evolving safety standards but also addresses technological advancements, ultimately driving innovation within the medical device industry while ensuring public health and safety.
How to Ensure Effective Medical Device Testing and Compliance in 2023
| Testing Category | Standards | Compliance Requirements | Frequency of Testing | Responsible Parties |
|---|---|---|---|---|
| Functional Testing | ISO 13485 | Documentation of test criteria and results | Before market release and annually | R&D and Quality Assurance teams |
| Safety Testing | IEC 60601 | Risk management file and compliance report | At product launch and every five years | Safety Engineering and Regulatory Affairs |
| Usability Testing | ISO 62366 | User feedback documentation and risk evaluation | User studies before launch | Product Design and Usability Specialists |
| Software Verification | IEC 62304 | Verification plan and quality metrics assessment | Continuous throughout software development lifecycle | Software Development and QA teams |
Key Regulations Impacting Medical Device Testing Procedures
Key regulations play a crucial role in shaping the testing procedures for medical devices, ensuring they meet safety and efficacy standards. In 2023, notable regulations such as the Medical Device Regulation (MDR) in the European Union and the Medical Device Safety Action Plan in the United States have reinforced the need for rigorous compliance. The MDR emphasizes a comprehensive assessment of risk management processes and clinical evaluations, pushing manufacturers to provide robust clinical evidence to support their device claims. This shifting landscape demands that organizations stay informed and proactive in adapting their testing methodologies to meet the evolving regulatory requirements.
Furthermore, the implementation of ISO standards, particularly ISO 13485 for quality management systems, continues to significantly influence medical device testing. This standard not only outlines the necessary requirements for establishing a quality system but also integrates risk management and post-market surveillance into the testing framework. By adhering to these regulations and standards, manufacturers can enhance product reliability and ensure that their medical devices are safe for public use. Effective collaboration between regulatory bodies, manufacturers, and testing laboratories is essential in navigating these complexities and maintaining compliance in a highly regulated environment.
Medical Device Testing Compliance in 2023
Step-by-Step Guide to Developing a Robust Testing Strategy
Developing a robust testing strategy for medical devices in 2023 requires a comprehensive understanding of regulatory standards and the integration of modern testing methodologies. According to a report by the Medical Device Innovation Consortium (MDIC), around 30% of medical device recalls are due to design flaws that could have been identified through rigorous testing. This statistic highlights the critical need for a well-defined testing framework that includes both pre-market and post-market assessments. By implementing a structured approach, companies can not only comply with regulations set by the FDA and EU MDR but also ensure the safety and efficacy of their devices.
A step-by-step testing strategy should incorporate risk management, user-centered design evaluations, and validated testing protocols. For instance, the International Organization for Standardization (ISO) emphasizes the importance of ISO 14971, which requires manufacturers to have a risk management process throughout the product lifecycle. Additionally, employing advanced simulation tools can enhance testing accuracy—reported to reduce development time by up to 50% according to a recent study from the Association for the Advancement of Medical Instrumentation (AAMI). By aligning testing strategies with these insights, companies can significantly improve compliance and ultimately enhance patient safety.
Best Practices for Data Collection and Analysis in Device Testing
In 2023, ensuring effective medical device testing and compliance is paramount, particularly when it comes to data collection and analysis. Medical device firms should adopt best practices that facilitate robust testing and reliable outcomes. One key practice is to leverage automated data collection tools that can streamline the gathering process while ensuring accuracy. This reduces human error and speeds up the analysis, allowing for quicker iterations and enhancements.
**Tips:** Consider implementing a centralized data management system that can integrate with existing devices. This not only enhances data visibility but also facilitates compliance with regulatory requirements. Furthermore, regular audits of the data collection methods can uncover inefficiencies and improve the overall testing framework.
Another important aspect is to invest in advanced analytical tools capable of real-time data analysis. These tools can provide deeper insights into device performance and user interactions, allowing manufacturers to make informed decisions. Additionally, collaboration with external testing partners can add an extra layer of expertise and objectivity, ensuring that testing processes meet industry standards and regulations effectively.
Navigating Post-Market Surveillance and Compliance Challenges
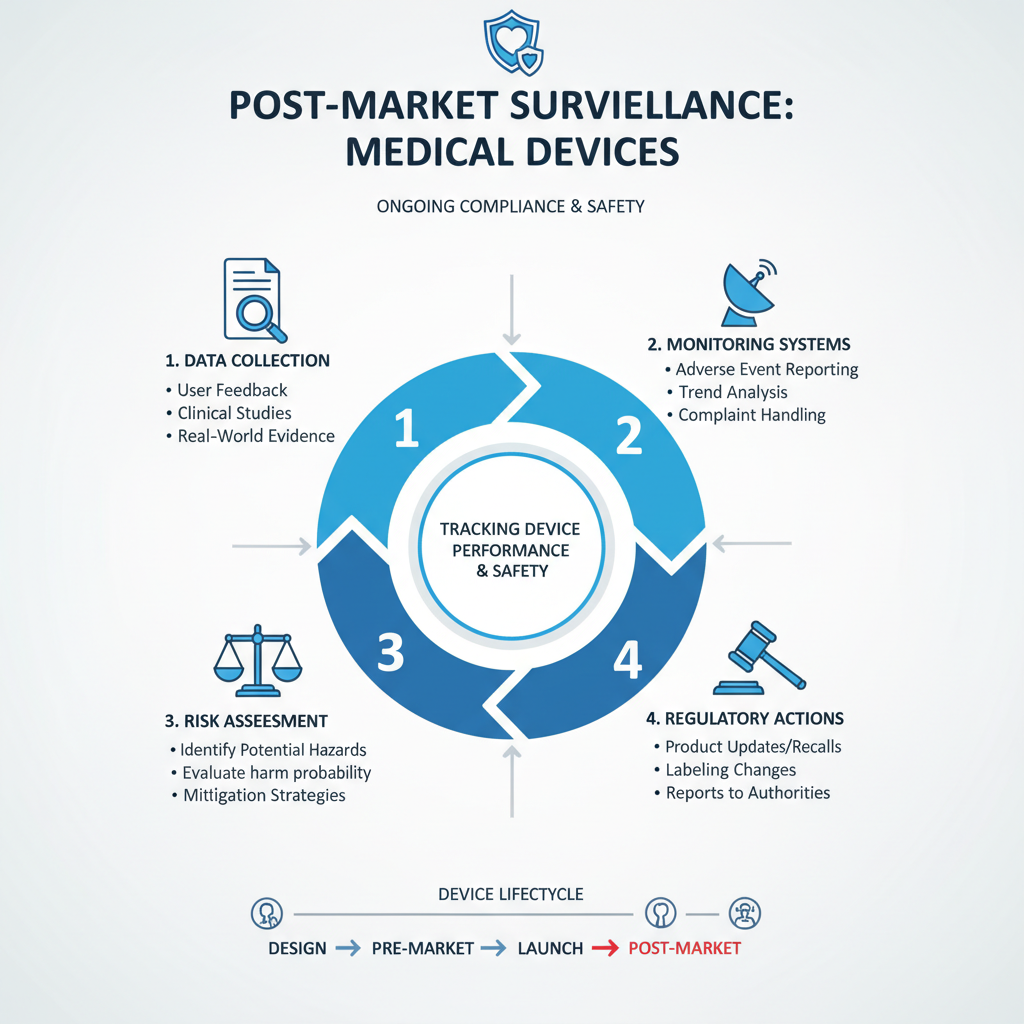
Navigating post-market surveillance in the realm of medical devices is crucial for ensuring ongoing compliance and safety. As products move beyond initial testing and into the hands of healthcare providers and patients, manufacturers must implement robust monitoring systems to track performance and report any adverse events. This entails collecting data from various sources, such as user feedback, clinical studies, and real-world evidence, to identify potential risks or product failures that may emerge after the device has entered the market.
Compliance challenges often arise in this context, particularly due to the evolving regulatory landscape. Manufacturers are required to stay abreast of changes in regulations, which may involve updating protocols for data collection and analysis or enhancing reporting mechanisms. Effective communication with regulatory bodies is essential, enabling timely responses to compliance issues and fostering trust in the data provided. By establishing a proactive approach to post-market surveillance, companies can refine their quality management systems and ensure their devices continue to meet safety standards, ultimately protecting patient health and safeguarding their market position.
Related Posts
-

Advantages of Medical Device Testing for Global Buyers
-

Challenges in Ensuring Quality in Medical Device Testing
-

How to Ensure Compliance in Medical Device Testing for Optimal Performance
-

Challenges in Medical Device Design that Could Affect Patient Outcomes
-

How to Accelerate Your Medical Device Development Process for Faster Market Entry
-

How to Navigate the Challenges of Medical Device Development in a Rapidly Evolving Market
